
Profile Name
Profile desiganation
Profile Content
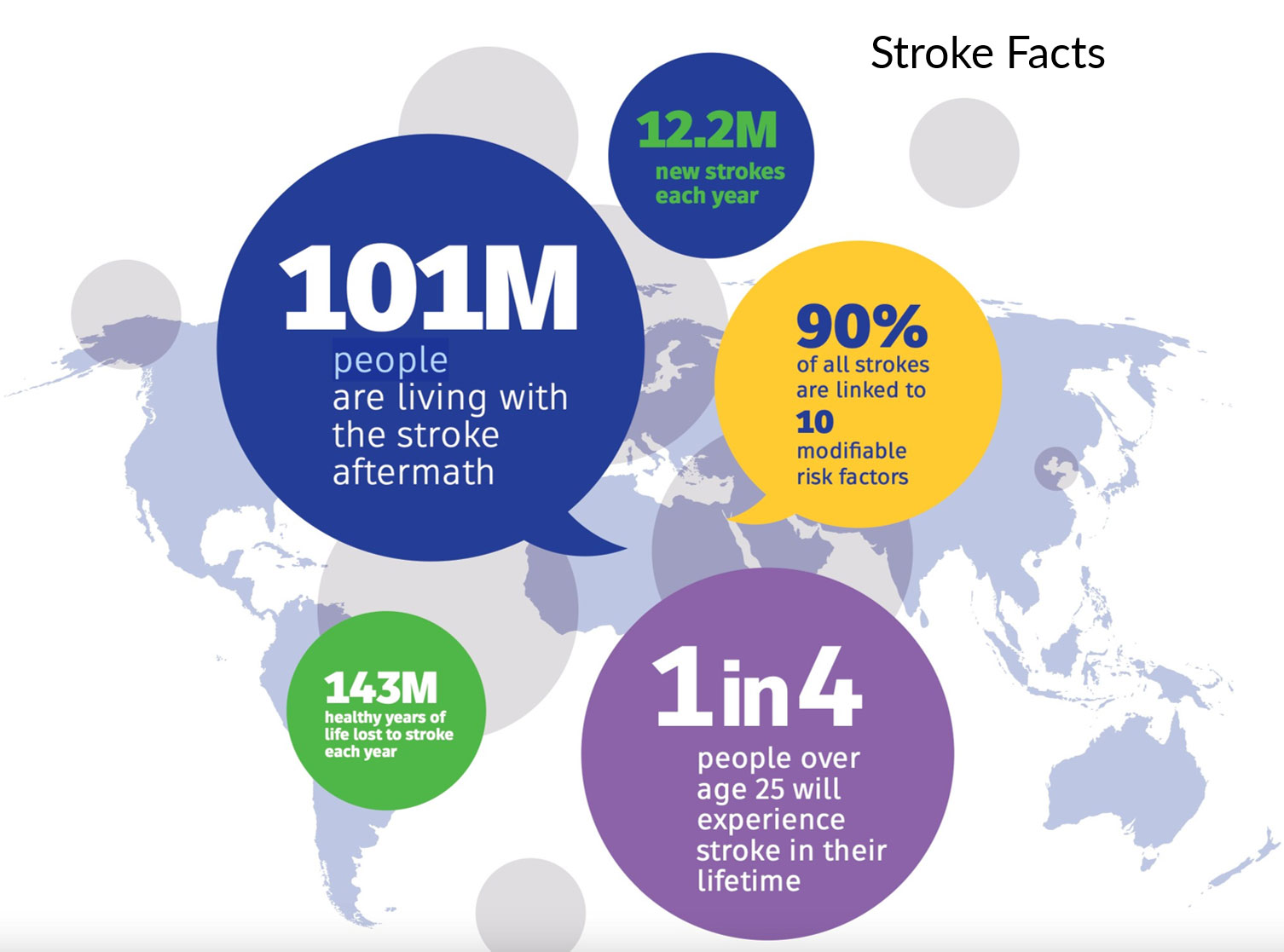
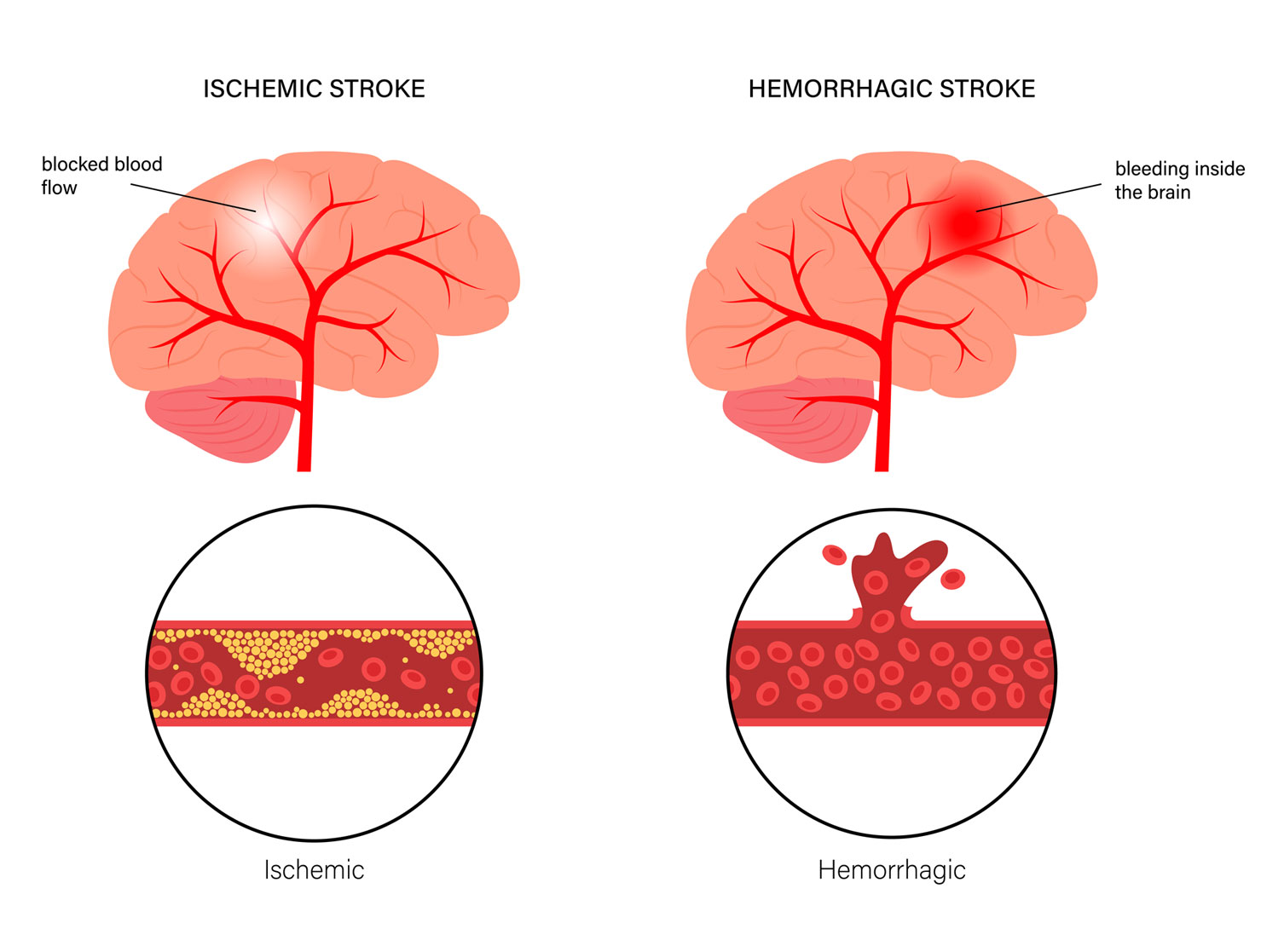
There are 12.2 million strokes every year globally. There are over 101 million patients with chronic stroke with ongoing disabilities. Eighty-seven percent of all strokes are ischemic, which are caused by blocked arteries. * There are no current therapies for chronic stroke, only rehabilitative post-stroke care to improve Quality of Life (QOL).
Stemedica has completed a Phase I/IIA clinical trial to assess the safety and efficacy of using adult bone-marrow derived mesenchymal stem cells for ischemic stroke patients.
The clinical trial was conducted at the University of California, San Diego with Michael Levy, M.D. as the principal investigator, at Gilbert Hospital under the leadership of Dr. Nabil Dib, MD and at the University of California Irvine under the leadership of Steven Cramer, MD.
The results showed preliminary improvement in all endpoints.
*From Center for Disease Control and Prevention’s “Stroke: Stroke Facts” published March 2015
Alzheimer's disease (AD) is a progressive neurodegenerative disorder that affects memory, cognition, and behavior. An estimated 7.2 million Americans age 65 and older are living with Alzheimer's. About 1 in 9 people age 65 and older (11%) haves Alzheimer's, and it is the sixth-leading cause of death across all ages in the United States. *
The FDA has approved a study:
Case reports show preliminary efficacy of SanoMSC in Alzheimer's
*From Center for Disease Control and Prevention’s “Healthy Aging: Alzheimer’s Disease” published March 2015.
COVID-19 is a contagious disease caused by the coronavirus SARS-CoV-2. In January 2020, the disease spread worldwide. Covid-19 can lead to acute respiratory distress syndrome (ARDS), a severe lung condition characterized by inflammation and fluid buildup in the lungs. This can cause severe shortness of breath and potentially require mechanical ventilation.
SanoMSC were used in numerous FDA approved emergency INDs with excellent results. The USFDA has approved a study:
: A Phase II Study in Patients With Moderate to Severe ARDS Due to COVID-19.
DCGI has also approved a Phase II IND in India for ARDS with Covid-19
There are 590 million diabetes patients globally and expected to rise to 643 million by 2030. Diabetes is the leading cause of death and morbidity. India has the 2nd highest number of patients with diabetes.
Over 1,200 patients with diabetes were treated with regular MSC or SanoMSC with excellent results as below:
MSC approved for Diabetes for commercial use by the Ministry of Health, KZ, Sept 2009
