
Profile Name
Profile desiganation
Profile Content
Ischemia-tolerant mesenchymal stem cells, or itMSCs, are “trophic” cells that move throughout the body and release growth hormones and proteins into injured tissue, saving the damaged cells from dying and establishing the ideal environment for the newly mobilized cells to multiply and heal. Sano Stemedica’s itMSCs (SanoMSC) are grown utilizing our in-house technology after being isolated from 1 single donated bone marrow.
Ischemia-tolerant neural stem cells, or itNSCs, are multipotent, largely undifferentiated cells that produce the primary phenotypes of nervous system cells. Donated brain tissue is used to extract Sano Stemedica’s itNSCs, which are subsequently grown utilizing our patented method.
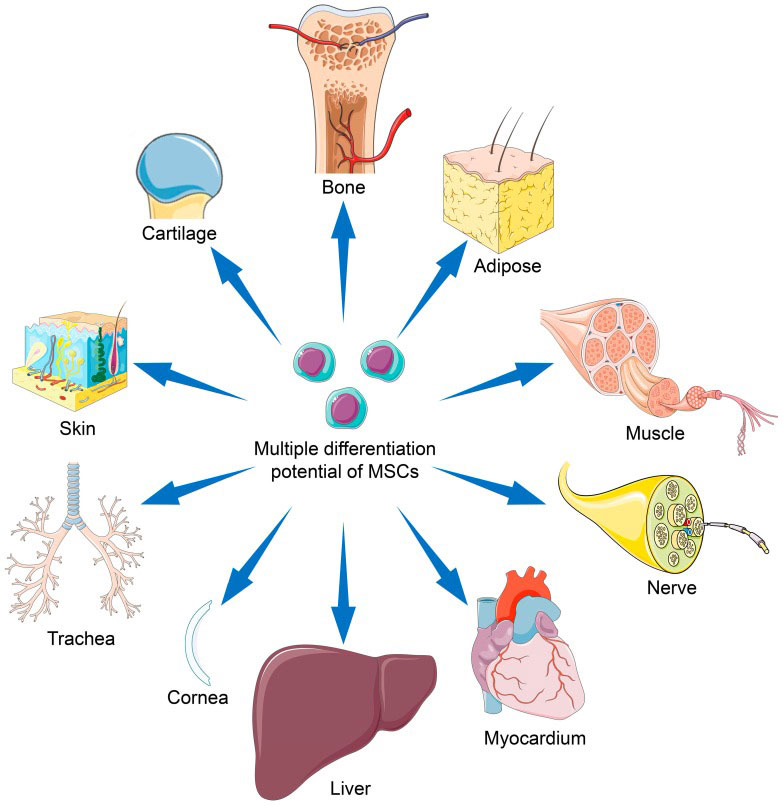
ItMSCs and itNSCs are produced by Sano Stemedica for use in FDA-approved clinical trials in the United States. Furthermore, we provide our progenitor cells to geographic distributors for use in research through ThermoFisher Scientific, our exclusive distribution partner, and for clinical trials that have been authorized by international regulatory bodies.
The following characteristics are among the unique qualities of Sano Stemedica’s itMSCs (SanoMSC) and itNSCs (SanoNSC):
Ischemia tolerant: To counteract ischemia circumstances, Sano Stemedica produces its progenitor cells in a hypoxic environment. ItMSCs, for instance, release more of the vital cytokines that promote healing, like VEGF and SDF-1. SDF-1 aids in the mobilization of the patient’s own progenitor cells, whereas VEGF is essential for the development of new blood vessels.
Immune privileged: Progenitor cells from Sano Stemedica lack the HLA-DR antigen proteins, which could lead to rejection. Using flow cytometry analysis, each batch’s lack of HLA-DR expression is verified. During treatment, no immunosuppressive medications are needed.
Documented safety: Endogenous viruses, adventitious agents, bacteria, mycoplasma, endotoxins, acute and chronic toxicity, and tumorigenicity are all thoroughly tested for in the cells.
Established purity: Sano Stemedica upholds strict standards for every relevant biological sign that denotes the purity of cells. Moreover, lot-to-lot repeatability is indicated by thorough batch testing.
Verified potency: Progenitor cells need to release the right growth factors, cytokines, and hormones in order to function well in vivo. Additionally, the progenitor cells must exhibit the capacity to develop into particular tissue types, such as cartilage, neurons, or bone. Before releasing its progenitor cells for therapeutic use, Sano Stemedica makes sure they fulfill the necessary requirements.
Stem cell therapies rely on autologous or allogeneic progenitor cells:
Sano Stemedica is dedicated to advancing stem cell therapies that transform lives. Our cutting-edge technology, rigorous standards, and scalable solutions position us as a leader in regenerative medicine.